Daxor is an innovative medical device company focused on blood volume measurement in patients who are critically ill or have heart failure. The company developed and now markets the BVA-100® (Blood Volume Analyzer), the first diagnostic blood test cleared by the U.S. FDA to provide safe, accurate, direct quantification of blood volume status and composition compared to patient-specific norms.
In the first half of the year, Daxor reported an increase in revenue of 29.6% in its diagnostic division, representing strong strides in the commercialization of its BVA-100 test. Each of the past two years, kit sales have risen over 18% year-over-year, driven by increasing awareness of the value of blood volume analysis. Importantly, there is reason to believe the trend will remain strong for the remainder of the year following Daxor’s $1.1 million contract with the Department of Defense for the development of a blood volume analyzer intended for combat casualty use. Additionally, there is clear empirical evidence to suggest the use of the BVA-100 to conduct blood volume analysis could have a meaningful impact on the treatment of patients with COVID-19.
Volume Is Vital
“You wouldn’t give someone insulin without knowing their blood glucose level, yet we give fluids and diuretics relying on indirect proxy measures that poorly correlate with circulating blood volume…”
Maintaining optimal blood volume is key to tissue perfusion and survival. Blood carries oxygen to tissues, and without oxygen, they die. There needs to be adequate blood volume and red blood cells to carry oxygen to keep cells alive and healthy. Inadequate or low blood volume ("hypovolemia") inhibits tissue perfusion and organ function. Hypovolemia can result in weakness, fatigue, fainting, dizziness, neurological impairment, shock, multi-system organ failure, and death. Patients with hypovolemia must be given fluids to restore their optimal volume, and the right balance between blood products and other fluids is essential.
Conversely, too much fluid ("hypervolemia") is also very detrimental to patients. Hypervolemia can result in heart failure, kidney failure, peripheral edema, ascites, dyspnea, and death. Knowing the patient’s optimal volume is vitally important.
Published studies from leading institutions such as the Cleveland Clinic, Yale University School of Medicine, and Columbia Presbyterian Medical Center demonstrate that patient care guided by direct blood volume analysis significantly improves multiple measures of outcomes in both heart failure and critical care. By understanding and treating the entire derangement, including total blood volume and red blood cell derangements, clinicians can more efficiently and effectively achieve euvolemia (normal volume), lowering mortality, reducing readmissions, and reducing the total cost of care.
Yet, physicians often face significant uncertainty regarding a patient’s optimal volume as they rely on indirect proxy measures that do not directly measure volume. Some methods are costly, invasive, and all lack sensitivity and specificity to the measurement of volume because they measure secondary effects such as blood pressure, hemodynamics, or clinical symptoms that are often misleading. Daxor’s BVA-100 test can effectively end the debate at the bedside. An accurate measurement of volume status that can more effectively guide treatment protocols can mean the difference between life and death for the patient.

Daxor’s Better Mousetrap
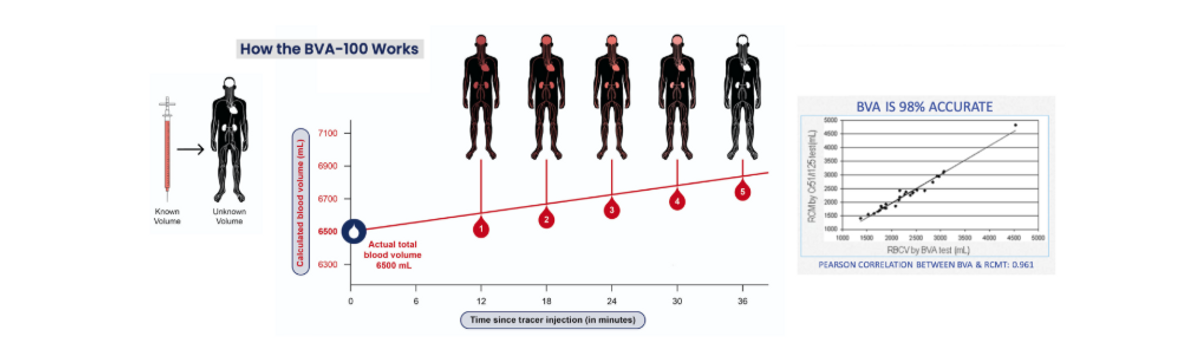
Daxor’s BVA-100 is a blood test with results available within 1-hour. An isotopic tracer, Volumex (Albumin I-131), is injected into the patient at the bedside. Once the tracer has fully circulated in the bloodstream, a series of small blood samples are drawn. The BVA-100 analyzer automatically calculates patient blood volume by comparing the concentration of undiluted tracer prior to injection to the tracer concentration diluted in the patient blood samples. This method is considered the “gold standard” for blood volume measurement. Clinical results show Daxor’s BVA-100 measures intravascular blood volume with an accuracy of 98%, with preliminary results possible in 30-minutes for emergency cases.
Quite simply, by delivering this critical information, it enables more precise diagnosis and treatment. Daxor’s BVA-100 test can be used to determine appropriate treatments and track efficacy, diagnose volume abnormalities associated with chronic illnesses or traumatic conditions, and assess risk before surgery. It is used in leading medical centers for evaluating and treating patients with heart failure, kidney failure, syncope, and to aid in fluid and blood transfusion management in the critical care unit. It also has been used to aid in the treatment of polycythemia, hypertension, anemia, chronic fatigue, and to assess risk before and during surgical procedures.
Growing Clinical Evidence of Very Significant Outcome Improvements with BVA
To date, the BVA-100 test has generated over 45,000 results and has been used in over 100 studies, drug trials, and case studies since its launch. Peer-reviewed studies by leading organizations including the Cleveland Clinic, Mayo Clinic, Yale University, Department of Veteran Affairs, and The Queen’s Medical Center have established the value of Daxor’s diagnostic, confirming that accurate blood volume measurement leads to better-informed physicians, better treatment strategies, improved patient outcomes, and better resource utilization. Daxor and independent work has validated the BVA-100 test as a diagnostic tool for the treatment of anemia, blood loss during surgery or trauma, chronic fatigue syndrome, hypertension, renal failure, and syncope of unclear etiology.
A particularly significant recent study reported on a 245 propensity-score match controlled cohort of hospitalized heart failure (HF) patients, whose inpatient care was guided by blood volume analysis. Results showed that use of Daxor’s BVA-100 test resulted in an 82% reduction in 30-day mortality (2.0% vs. 11.1%), an 86% reduction in 1-year mortality (4.9% vs. 35.5%), and a 56% reduction in 30-day hospital readmission (12.2% vs. 27.7%) with a P<0.001 (very statistically significant). These results are published in JACC: Heart Failure (Strobeck et al., 2018).
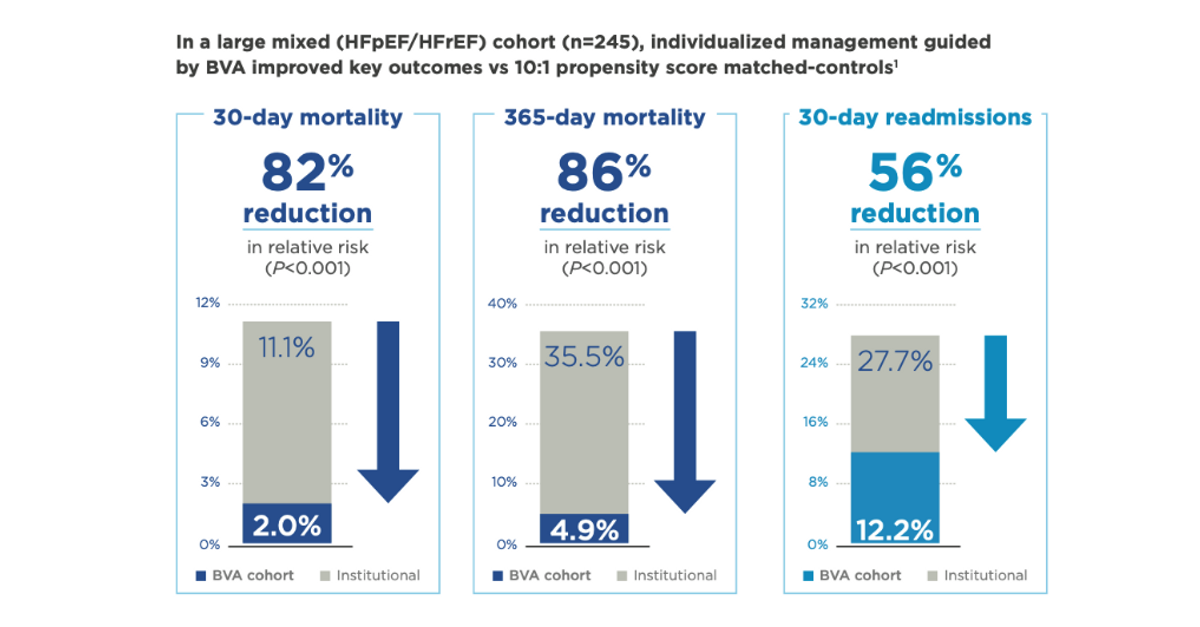
Expanding treatment of patients with heart failure is a tremendous opportunity for Daxor. According to the U.S. CDC, an estimated 6.2 million Americans are living with heart failure today, and there are an estimated one million admissions to the hospital each year for the disease. Heart failure costs the nation over $30 billion in direct and indirect costs. The data above suggests that over 35% of hospitalized patients will die (actual deaths in 2018: 379,800). Daxor’s technology helps improve outcomes at hospitals with its technology in place, and clinical evidence shows a reduction of mortality from over 35% to less than 5% is achievable.
Sepsis is the number one leading cause of deaths in hospitals and hospital readmissions at a cost of $27 billion per year. A separate Prospective Randomized Control Clinical Trial in which 100 critically ill surgical patients with septic shock, severe sepsis, severe respiratory failure, and/or cardiovascular collapse admitted to the ICU showed that the use of the BVA-100 test resulted in a 66.6% reduction in mortality vs. conventional care (8% vs. 24%). These data are published in Shock (Yu M et al., 2011). BVA is showing its value in both the HF and Critical Care world as managing patient blood volume derangements is central to improving outcomes.
These are powerful and clinically meaningful results. Any hospital ER or ICU should have high motivation to use the BVA-100 test for a range of conditions -- the business model follows the validated razor-razorblade model. Daxor sells (or leases) the device, which sells for roughly $85,000, to the hospital, and then sells one-time consumable test kits for $325 each. The BVA-100 test is reimbursed by both Medicare and private insurances in both the inpatient and outpatient settings, with separate billing codes for the tracer, the test, the consumables, and the components. Reimbursement is a crucial advantage for the company, as many diagnostic products never achieve reimbursement.
Notably, an analysis of the cost-effectiveness of the BVA-100 test from a 30-year simulation was presented at the Heart Failure Society of America’s (HFSA) Virtual Annual Scientific Meeting 2020. The ICER analysis shows the cost of the BVA-100 test is approximately $10,700 per patient life year gained, well below the threshold of $50,000 generally recognized as “very good value.” The product should become even more attractive to hospitals and patient care centers as the U.S. moves more and more towards “pay-for-outcome” healthcare policies instead of the old “fee-for-service” model that has become costly and outdated.
BVA-100 Is Particularly Important During COVID-19 -- Prospective Multi-Center Trial Initiated
A lot of companies have been investigating their novel therapeutics or devices for the treatment of COVID-19. Many are experimental, without FDA approval, and have sought emergency exemptions from FDA and are repurposed in hopes they will be antagonistic to the COVID-19 virus. Conversely, Daxor’s BVA technology has peer-reviewed published data from a randomized controlled trial demonstrating reductions in mortality for patients in the ICU suffering from acute respiratory distress syndrome and septic shock, the same complications that arise from COVID-19 infection for ICU patients.
In the randomized clinical trial (RCT), clinicians noted a 66% reduction in mortality demonstrated in the ICU in patients with suffering predominantly from septic shock, severe sepsis, and severe respiratory failure - a very similar presentation to patients with COVID-19 - use of BVA-100 test also resulted in a reduction in ventilator use and ICU length of stay. The BVA test was so useful that in 44% of the tests the care teams changed treatment fluid strategy solely as a result of the data from the test. This is an extraordinarily high actionability level as most ICU test-results lead to no change in treatment strategy.
Daxor’s BVA-100 test also provides a unique metric - the albumin transudation slope - an indicator of the rate at which fluids may be escaping the intravascular space, which is a leading predictor of ICU mortality. Endothelial damage in the vascular structure is predominantly where the SARS-Cov2 attacks. The virus's assault on the vascular system is what leads to capillary leak and fluid imbalance. A high leak rate detection is key to understanding the disease process, giving physicians a true understanding of the status of the patient.
For example, a declining leak rate would indicate that the patient is improving versus a patient with a sustained or worsening leak rate. There is currently no other accurate way of directly measuring intravascular volume and red cell deviations of patients and no other test to give insights into the capillary leak rate than the BVA-100 test. Daxor is working with leading medical centers to initiate a clinical trial using the BVA-100 test in hospitalized patients with COVID-19. NYU Langone Health in New York City is the first site to enroll patients in this clinical study.
To survive COVID-19, patients may be given ventilator support to get oxygen to the lungs, but the right blood volume is crucial to ensure that red cells carry oxygen to the tissues. Without adequate blood volume to carry oxygen, the heart and the ventilator are useless.
Daxor’s BVA-100 test allows physicians to immediately adjust COVID-19 patients to the optimal blood volume by enabling them to see if a patient is too under or over resuscitated. Correct fluid adjustments ensure circulatory integrity and optimal tissue perfusion. In addition, the test will give the leak rate of the patient conveying critical insights into the disease state of the patient as well and help triage patients. That may, finally, give doctors the edge they need to help curb the death rate from this horrible pandemic.
Forward-Looking Statements
Certain statements in this release may include forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including without limitation, statements regarding the impact of hiring sales staff and expansion of our distribution channels. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this release, including, without limitation, those risks associated with our post-market clinical data collection activities, benefits of our products to patients, our expectations with respect to product development and commercialization efforts, our ability to increase market and physician acceptance of our products, potentially competitive product offerings, intellectual property protection, FDA regulatory actions, our ability to integrate acquired businesses, our expectations regarding anticipated synergies with and benefits from acquired businesses, and additional other risks and uncertainties described in our filings with the SEC. Forward-looking statements speak only as of the date when made. Daxor does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.